Decision making under uncertainty is a challenge we all face every day in both our personal and professional lives.
Our team has addressed the topics of uncertainty and risk previously in a webinar, Automating Under Uncertainty as well as an article published in ondrug delivery.
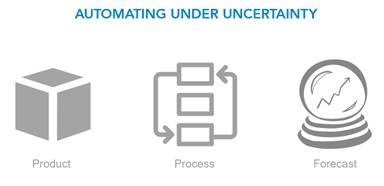
In these publications we focused on three sources of uncertainty:
1. Product
2. Process
3. Sales forecast
While we touched on external factors in the sales forecast, our discussion was focused largely on the voice of the customer and changing tides in the marketplace. What we are observing now, particularly in the Life Sciences world, is external and unforeseen factors impacting the decisions we make in our working lives. The most obvious example today is how, many of us in the life sciences (or associated)fields are working tirelessly to be part of the solution to the global COVID-19 crisis, which is layering a fourth factor (environmental) onto the sources of uncertainty in our professional lives. I’ll talk more about the causes of uncertainty and the impact they have on manufacturing’s ability to scale in my next webinar, 3 Practices to Reduce Risk when Automating Under Uncertainty.
“By definition there are no longer any ‘best practices’ that can guarantee success for an organization; there are only good practices that we can stumble upon through experimentation.” – Alison Randel
While COVID-19 has accelerated change in a number of areas within the industry, the primary principles for addressing uncertainty haven’t changed.
Here are the 3 primary practices that I recommend to help minimize risk in an undefined circumstance:
1. Start with the end in mind
2. Select solid foundational technologies
3. Minimize customization
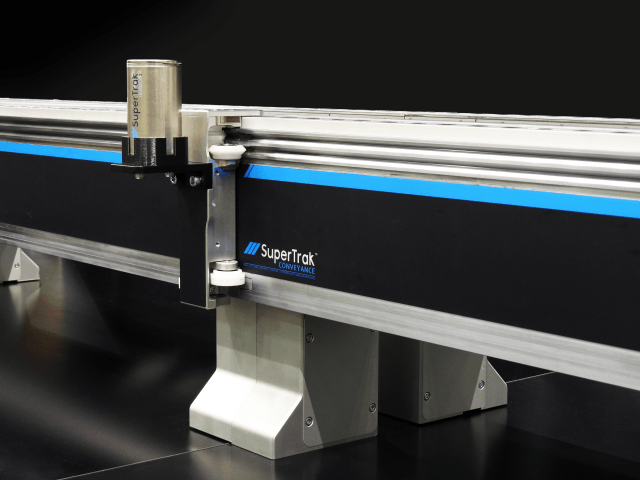
SuperTrak’s parent company, ATS Automation, put these principles into practice when executing a large automation program utilizing the SuperTrak CONVEYANCE™ platform for Tessy Plastics. Having a clear vision of what we needed to do, utilizing proven technologies, and re-configuring stations rather than redesigning them have empowered the deployment of a system at a pace previously thought unachievable.
Let’s take a look at each practice.
Start with the end in mind
Starting with the end in mind allows for more effective process development, especially in the regulated world, because as we invest in equipment to support each of the various volumes (validation, clinical trial, human trials) we want to be sure that we are building our knowledge base in support of the system which will ultimately manufacture the good. This practice makes regulatory steps more straightforward and streamlined at each step, while also driving the effectiveness of the specialists needed to build the manufacturing process.
Select solid foundational technologies
Sound foundational technologies are useful in the life sciences market because they provide building blocks to anchor your overall system design. Consistency, reliability, and cleanliness are vital requirements as we assemble life-critical devices. Driving minimal floor space within a cleanroom, high throughput rates, and fine process control are enablers for the investment in automation. There is a balance to be had in regulated industries between trusted components that we’ve used in certification and the modernization of technology through new standards. Leaders in the space are finding means of driving innovation while respecting the knowledge base that has been built over time, which is often done through new strategic partnerships such as the one the SuperTrak team recently formed with Vention.
Minimize customization
Reconfiguring rather than re-designing helps to reduce the risk in an already challenging field. Adding market and environmental risk into the Life Sciences field can impact the business case for an automation investment. However, a reduction in NRE by careful consideration of market available technologies can help to improve the value of the investment. Our team has noted that standardization is a trend that has been steadily increasing within our partner base and one that we are observing continuing to accelerate. With growing uncertainty around the market, labor force, and other external factors, standard technologies allow for higher agility and lower risk on the manufacturing floor.
With the above in mind, we have re-tooled our automating under uncertainty webinar to focus on the specific challenges of life science manufacturers and what they can do to reduce their automation risks. 3 Practices to Reduce Risk when Automating Under Uncertainty leverages decades of experience as well as a broad understanding of current global trends to walk you through how a focus on configuration, not customization can truly help you reduce your risks while you invest in your automation.