Medical Device Manufacturing
Increase Production Without Increasing Footprint
Having flexible automation that you can develop as you go is critically important.
The SuperTrak CONVEYANCE™ platform offers modular designs to grow with you and your pharmaceutical and medical device machinery and develops with your needs as you move through the regulatory process – from development to validation and production.
This functionality allows medical, pharmaceutical, and life science manufacturers the ability to comply with strict regulations throughout the different phases of the automation process.

Increase Production Without Increasing Footprint
While your pharmaceutical production and medical device production demands may be increasing, floorspace can come at a premium cost, making expansion of your current process unsustainable.
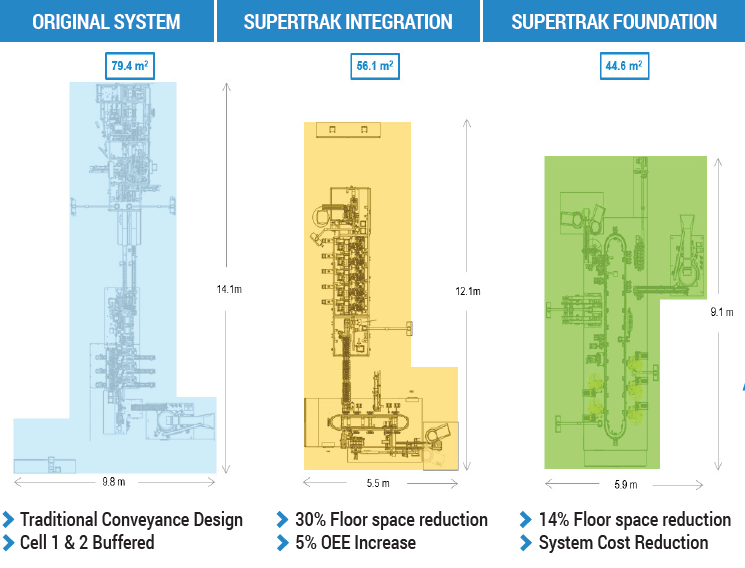
The SuperTrak CONVEYANCE™ platform offers integrated functionality; manufacturers can achieve high-performance in the minimum amount of space by reducing tooling and station redundancies. The platform also offers various configuration options to meet your unique space challenges. One medical device manufacturer was able to gain back 44% of their floorspace by using SuperTrak CONVEYANCE™.
Read more about their story and automation in the life sciences industry here.
Cleanroom Manufacturing
SuperTrak CONVEYANCE™ has been developed over the past 20 years with the medical and pharmaceutical industries in mind.
With few moving parts, the SuperTrak CONVEYANCE™ platform is easy to maintain and generates very little particulate matter, keeping your operations clean and moving quickly. Minimal moving parts lead to low maintenance and results in minimal downtimes and increased throughput.
Quiet Operation
This Smart Conveyance system is also quiet – operating at > 75dBA – making it easier for those working in and around the automation system for long periods of time.
The main reasons that the SuperTrak GEN3™ system is quiet are:
- The SuperTrak GEN3™ system has very few moving parts. Each shuttle has 4 plastic rollers/wheels that provide low friction and a dampened rolling sound as they ride on the steel rail system.
- The built-in collision avoidance ensures no shuttle to shuttle contact or banging noise.
- There are no mechanical stop mechanisms required to precisely stop and locate a shuttle at a process station.
See more frequently asked questions here.
Resources
8 Ways SuperTrak CONVEYANCE ™ Helps Life Science Manufacturers Uncover Automation Efficiencies
As a manufacturer in the life sciences space, you deal with a lot of uncertainties that can impact how you choose to develop your automation. During the product development phase, requirements often change, during the scaling-up phase, you must adapt to changing market conditions and may be required to alter your processes or throughput. While these changes may be unavoidable, one thing remains certain, …
View BlogSuperTrak CONVEYANCE™ – Features & Applications
Real-life manufacturing applications highlight how the SuperTrak CONVEYANCE™ platform allows engineers and manufacturers the ability to overcome common manufacturing challenges related to factory footprint, scaling, market uncertainty, tight project timelines and re-deployability.
Watch VideoReducing Floorspace by 44%
How one manufacturing company gained back floor space and increased throughput. You know that in today’s manufacturing space you need to be flexible enough to adapt quickly. You need to efficiently adapt to changing market demands. Likely, you’ve already tried to figure out how to effectively expand your production lines as demand has increased …
Read Case Study
