To be successful, you need to deliver on your project commitments to your management team. We have the same dedication to project delivery success. You can count on our teams to support your project delivery needs from project management to final signoff at your installation site.
Project management is ultimately about people getting things done. Successful project execution is, in part, a result of a persistent focus on project management discipline. It’s key to set the course for the project team, define the success parameters, and nurture the relationship with you and your team for the duration of the partnership. At ATS Life Sciences, our highly skilled and experienced project managers have a proven track record of successfully delivering complex programs including the following.
- Planning and coordinating all necessary tasks
- Securing necessary resources
- Mitigating risk
- Monitoring, navigating, and iterating to accommodate dynamic project conditions
- Maintaining open, respectful, and collaborative lines of communication
Working with our project management team, you can be confident we will internalize and own your requirements, advocate for your project, and strive for an exceptional project outcome.
Operations keeps the lights on, strategy provides a light at the end of the tunnel, but project management is the train engine that moves the organization forward.
Joy Gumz, Project Management and Quality Assurance Consultant and Author
As a project progresses, the ease of making a change decreases and the cost associated with the change often increases. This is because as time passes, we have more invested and change means discarding, redoing, or adding to at least some of what we have executed. The trick is to identify risk early and mitigate it quickly. Our experienced team will help you put plans in place to acknowledge and avoid significant risks to the project.
Our project teams are frequently asked to solve automation problems that haven’t been solved before. Starting with an understanding of clear requirements, we leverage our technology expertise and product assembly, inspection, and testing know-how to inform our ideas, and construct and demonstrate a conceptual solution. To gain confidence, our team of technical experts, staff scientists, and suppliers draw on our previous experience to do the following and more.
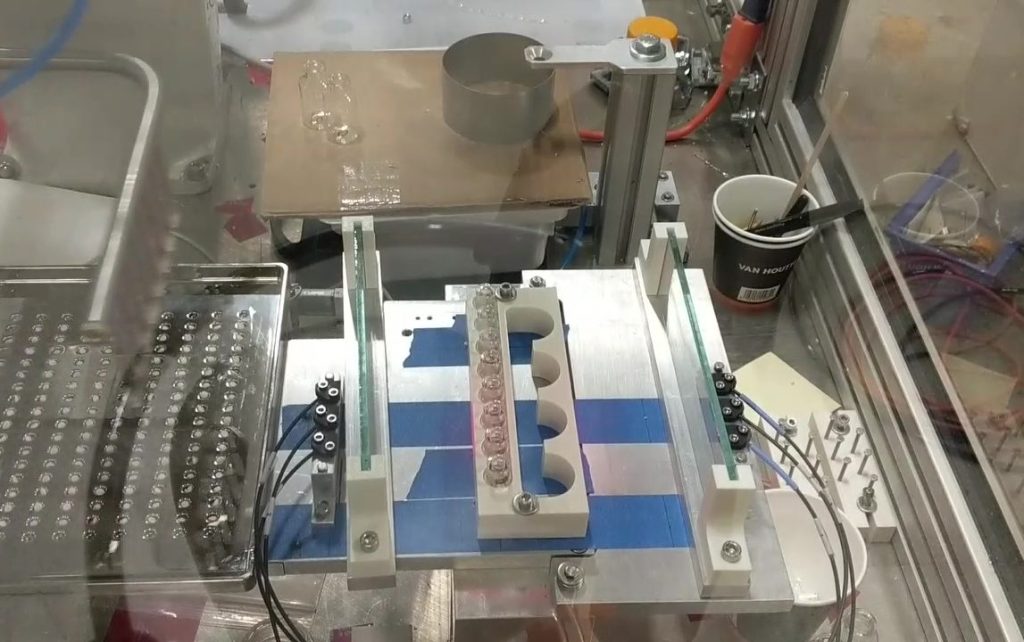
- Design proof-of-principle studies
- Specify prototypes
- Execute design of experiments
- Perform repeatability and reproducibility testing
In a regulated environment, complying with good manufacturing practices is essential requiring rigour and discipline. We’ve built this same focus into our engineering processes as a complement to your regulatory processes and needs.
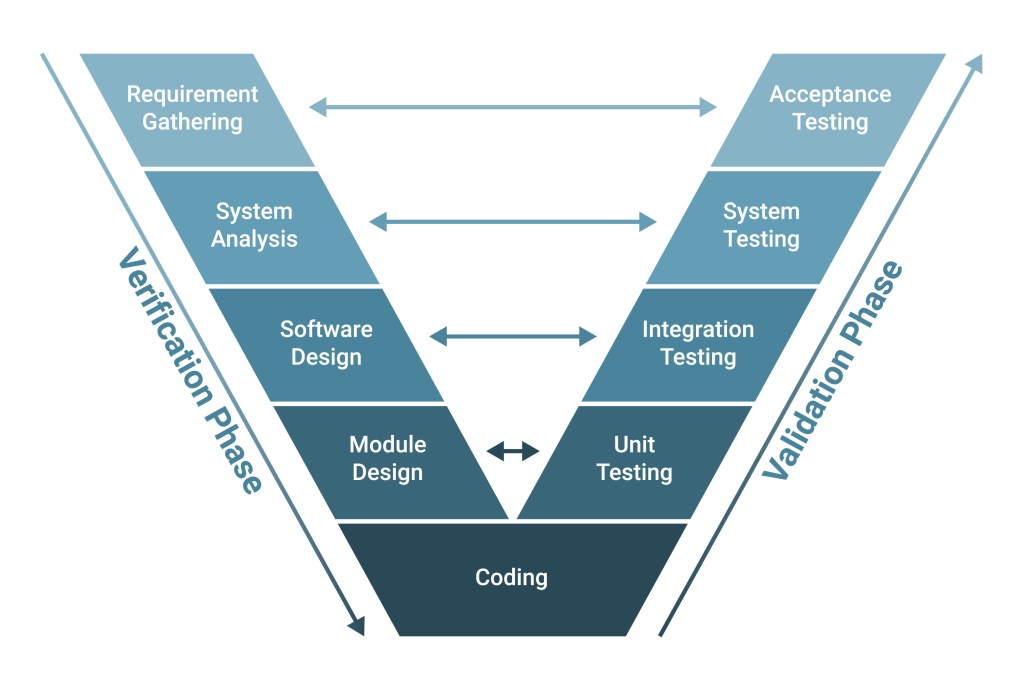
- We consult with you to identify critical-to-quality attributes so we know where to focus our GMP efforts.
- We build and maintain a trace matrix to document the evolution from your user requirements to final testing, including validation.
- We design our solutions to meet your GMP needs and outline them in a basis of design document before design begins.
- We partner with you through a rigorous design review process, and all requests and actions are formally recorded and closed.
- We follow a formal change control procedure to appropriately document, review, and approve requested.
- We test to confirm that the function satisfies the design intent as we integrate your system’s various elements.

We have a performance-driven, vertically integrated supply chain with a global presence. Our end-to-end capabilities include dedicated supplier management, value engineering, and value analysis services. Our vendor preselection program, auditing system, and annual report cards help ensure we are working with reputable, reliable, and responsible supplier partners. What does this mean to you? With multifaceted automation market experience, we are able to source the right products at the right prices in the right geographical area for your immediate project and operational needs and post-installation maintenance and upkeep needs.
From commissioning and qualification planning to final reports, we will work closely with you and your team to understand your unique regulatory environment and deliver a fully compliant system. This includes the preparation and execution of factory and site acceptance test protocols, installation and operation qualification protocols, and trace matrices, using our own pre-established templates or yours. Once testing is complete, you will have the necessary objective evidence to prove your user requirements have been met.
Fabrication, assembly, and integration of every system is the science of what we do. The art is in the application of our experience, collected over thousands of projects, to the fine-tuning and installation of your final product. Our artists turn what might otherwise be an ordinary machine into a superior solution.
Our people are the core of our business; protecting their safety and health and the environment is a fundamental business priority. Our corporate values are key to the ATS Sustainability program, which includes Health and Wellbeing and Safety Culture, where we support our people to foster a safe, positive, and inclusive workplace where everyone is respected and given the opportunity to do their best.

From leadership to employees at all of our sites, everyone is held accountable to themselves and each other to work in a safe and environmentally responsible manner. We work hard to maintain a culture of trust and transparency, so our staff are comfortable and confident in dialoguing about risks to safety and the environment. By empowering our teams to engage in the workplace in real time in risk assessment, inspections, and job safety analysis, we identify, assess, and control potential hazards, with the belief that all incidents can and must be prevented. Zero incidents are possible.
Our commitment doesn’t stop there. Our responsibility to prevent injury and protect the environment extends to our visitors and contractors as well, who all must meet our safe work expectations and practices. Everyone must go home safe and be well.





